Women with HIV are more likely to develop cervical cancer: Why?
You are here

Cervical cancer screening is low in many areas:
Over the years, through sc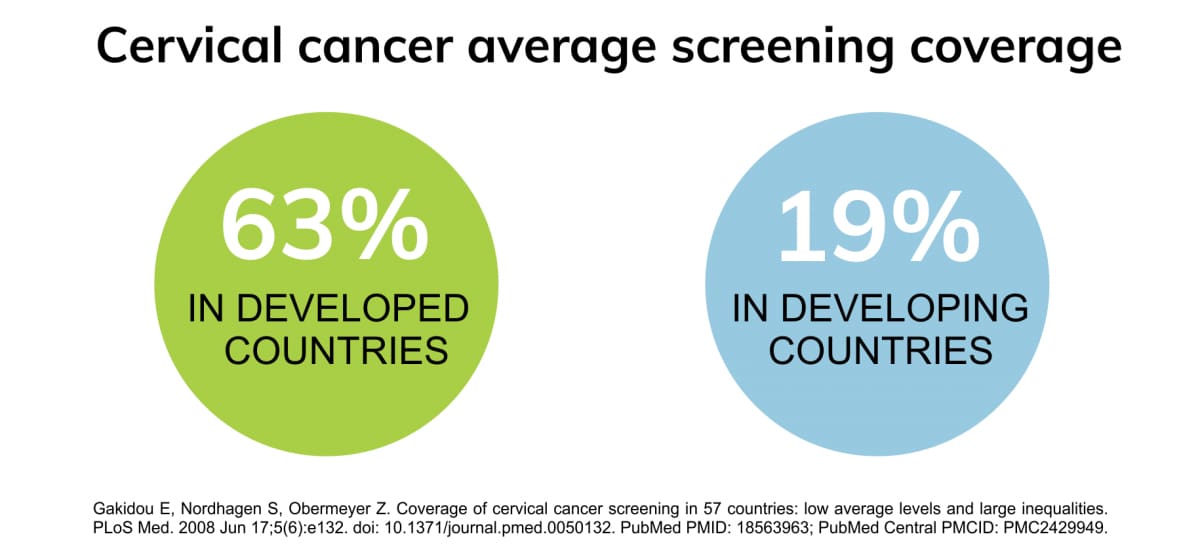 reening and vaccination, cervical cancer incidence as well as mortality rates have declined in many high-income countries. Yet, every year, more than 260,000 women die due to the disease, out of which, almost 90% occur in low- and middle-income regions, including areas of Africa, Asia, Latin America and the Caribbean (1). Primary reasons for the disparity include poor screening and vaccination uptake in these areas.
reening and vaccination, cervical cancer incidence as well as mortality rates have declined in many high-income countries. Yet, every year, more than 260,000 women die due to the disease, out of which, almost 90% occur in low- and middle-income regions, including areas of Africa, Asia, Latin America and the Caribbean (1). Primary reasons for the disparity include poor screening and vaccination uptake in these areas.
HIV positive women are more likely to develop cervical cancer:
It is well-established that high-risk human papillomavirus (hrHPV) strains are a major contributor to cervical cancer (1). While most HPV infections cause no harm and disappear naturally overtime in healthy individuals, women with compromised immune conditions such as Human immunodeficiency virus (HIV), are more likely to develop persistent HPV infections (1,2).

This higher risk is a result of several factors; women with HIV have an increased risk of acquiring HPV, lower regression of pre-cancer lesions, as well as more chances of cancer recurrence after treatment (3).
Further, in HIV-positive patients, HPV-related tumors tend to occur at a younger age and at a more advanced stage, highlighting the poor clearance of HPV infections in those infected with HIV (4).
Given the synergy between the two infections, it is critical that women with HIV are screened regularly for HPV. Guidelines by the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) recommend that newly diagnosed HIV-positive women undergo their first cervical cancer screening session at the time they receive their HIV health status and are sexually active (1,2). A repeat test is recommended after 6 or 12 months. Later, routine screening can be scheduled every 3 years, after three consecutive negative results. For women older than 30, with abnormal cytology or HPV test results, additional measures such as colposcopy and biopsies may be required (2).
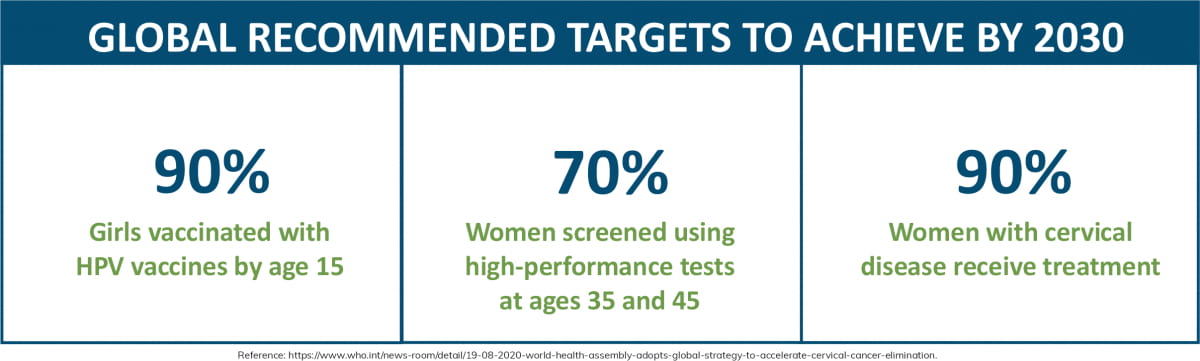
Importance of screening and potential of urine as a sample type:

To reduce preventable cervical cancer deaths, methods to increase awareness through health education and improve HPV vaccination and cervical cancer screening uptake (1). The WHO aims to achieve global targets by 2030 to eliminate the cancer type.
Cytology-based Pap smears are the traditional screening approach, which look for abnormal cervical cells. Despite the benefits, many women are often reluctant to undergo a Pap smear as the procedure is invasive and associated with physical discomfort. Additionally, religion, culture, inconvenient clinic hours, and/or lack of transportation can also influence decision of testing (5,6).
Therefore, improving screening uptake is necessary. Most low- and middle-income countries, which have the most women living with HIV, have poor screening and vaccination uptake(1,7). For example, in areas with high HIV prevalence, cervical cancer rates linked to HIV can be as high as 40% in some countries (3). In this population especially, alternative methods are critical.  HPV testing on self-collected samples is becoming increasingly acceptable and can be used as an additional strategy to reach women who do not participate in regular screening programs (8). HPV can be detected in urine (9) and offers several benefits.
HPV testing on self-collected samples is becoming increasingly acceptable and can be used as an additional strategy to reach women who do not participate in regular screening programs (8). HPV can be detected in urine (9) and offers several benefits.
Women have shown a significantly higher preference to urine as a sample type for hrHPV testing in comparison to brush-based cervical-vaginal self-sampling methods (10,11). Urine is also preferred as it non-invasive and suitable for home-collection (10,12). Recent studies confirm similar sensitivities between urine and clinician-taken smears as well as brush-based self-sampling for detecting high-grade lesions, (9,12), further highlighting the potential of urine as a sample type.
To know more about urine as a preferred sample type for HPV detection, read our blog.
Colli-Pee® - An innovative urine collection solution:
First-void urine (initial urine flow) contains higher concentrations of HPV DNA than a midstream urine sample (13,14), which is necessary for improved diagnostic accuracy. For this reason, Novosanis developed Colli-Pee®, a urine collection device, which allows for standardized, volumetric collection of first-void urine. Additionally, for HPV testing, a urine preservative is recommended to improve stability of the sample. Colli-Pee® can be pre-filled with Novosanis non-toxic proprietary UCM.
Read more about Colli-Pee® in HPV-based cervical cancer screening.
Read more about the journey of Colli-Pee.
Conclusion:
As women living with HIV are more at risk to develop invasive cervical cancer, regular screening for the cancer type is even more critical in this population. HIV rates in adolescent girls and young women are particularly high in areas where cervical cancer screening is significantly low. In these regions, that have poor access to essential health services, urine as a sample type for HPV detection offers great potential to reach more women,.
References:
(1) https://www.unaids.org/sites/default/files/media_asset/JC2851_HPV-HIV-cervicalcancer_en.pdf
(2) PMID: 28819634
(3) WHO releases new estimates of the global burden of cervical cancer associated with HIV
(4) PMID: 28368072.
(5) PMID: 23618210
(6) DOI: 10.1080/21642850.2020.1798239
(7) PMID: 30254961
(8) PMID: 30195193
(9) PMID: 25690816
(10) PMID: 28689156.
(11) PMID: 32212991
(12) PMID: 28391609.
(13) PMID: 24916950
(14) PMID: 30452931
