USA
You are here
Our products
Colli-Pee®: An innovative solution for non-invasive urine collection and preservation
Colli-Pee®, is a patented award-winning urine collection device. Colli-Pee allows volumetric and standardized collection of first-void urine. In 2014, Novosanis launched the Colli-Pee platform, today the platform contains a wide range of volumes from small to larger for standardized urine collected 4 mL up to 40 mL offering flexibility to meet the collection for different research and diagnostic applications.
Urine collection through Colli-Pee is easy, comfortable, and more accurate to capture first void urine than a regular urine cup (1).
The Colli-Pee device architecture enables immediate mixing of the sample with a urine preservative, improving stability of the urine specimen.
On this page:
- Our products
- Colli-Pee and first-void urine
- How does Colli-Pee work?
- Product Overview
- Home collection
- Colli-Pee Postal Kits for home collection
- Quality
- References
Colli-Pee and first-void urine
Urine sampling: an emerging sample type
The most common liquid biopsy is blood, however blood sampling has several limitations:
- Blood is a complex, protein-rich sample type that often requires additional processing, which can interfere with biomarker measurements
- Blood is relatively invasive
- Blood can pose a risk of infection for both the patient and the caregiver (2)
Alternatively, urine sampling has multiple benefits:
- Urine is easily accessible and available in larger quantities
- Urine sampling is non-invasive
- Urine can be collected easily by an individual at home
Urine sampling offers several benefits, however, not all urine fractions are the same. First-void urine is considered the first 20 mL to 30 mL of urine flow.
Collecting an accurate first-void urine sample with a standard urine cup can be awkward, messy, and inconvenient for the user. For this reason, Novosanis developed Colli-Pee.

Urine sampling advantages
Urine fractions – First-void urine
First-void urine, also known as first-catch or first-pass urine is collected at any time of the day and is typically referred to the first 20 mL to 30 mL of urine flush. A first-void urine specimen has shown to contain higher concentrations of sexually transmitted infections (STI)- related DNA than other fractions (3,4). Additionally, first-void urine can be important to identify cancer biomarkers (2).
When to use first-void urine
First-void urine contains more DNA and RNA, as well as other analytes, than other fractions such as a random or midstream urine sample (5).
For urological applications, a urine specimen is in many situations the preferred liquid biopsy source.
Given the importance of capturing first-void urine for improved accuracy and test results, Novosanis developed Colli-Pee, a first-void urine collection device. The unique design of the device allows easy collection of first-void urine and improved sample preservation, which can be challenging and difficult with a regular urine cup.
"I am convinced that the clinical and diagnostic information present in a first-void urine sample still is greatly underestimated. We confirmed that first-void urine is an interesting sample to monitor HPV vaccination programs; possibilities in cervical cancer screening programs are being explored. The non-invasive character of urine sampling, with option of home collection, will definitely help to enroll underserved women in cervical cancer screening and follow-up programs across the world."
Prof. Alex Vorsters
HPV VAXINFECTIO, University of Antwerp, Belgium
"Self-collected urine, through a first-void urine collection device, has the potential to increase uptake of cervical cancer screening. Urine collection can be more comfortable and more socially acceptable for some women who are reluctant to perform a vaginal examination”
Prof. Clementina Cocuzza
Department of Medicine and Surgery, University of Milan-Bicocca, Italy
How does Colli-Pee work?
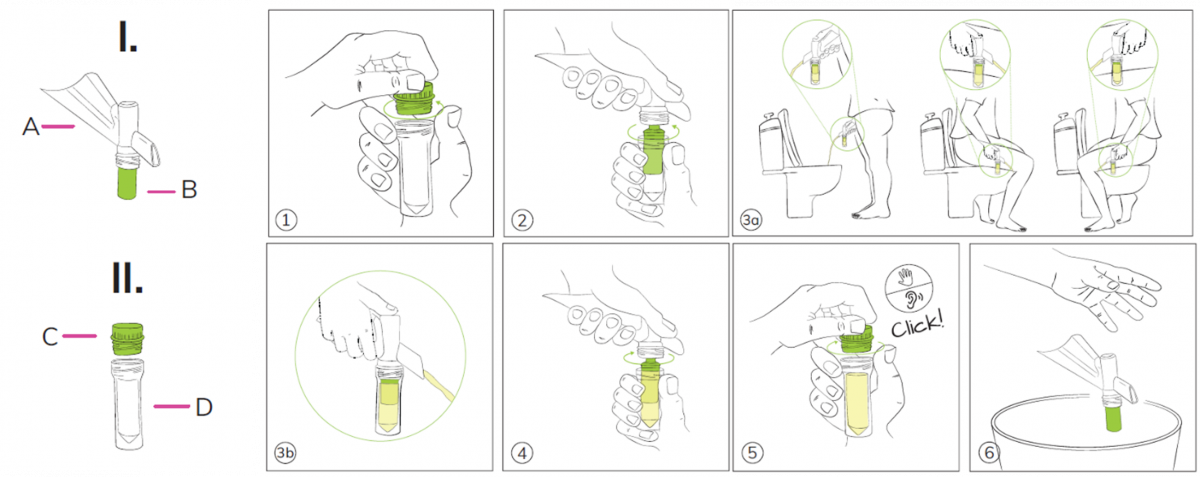
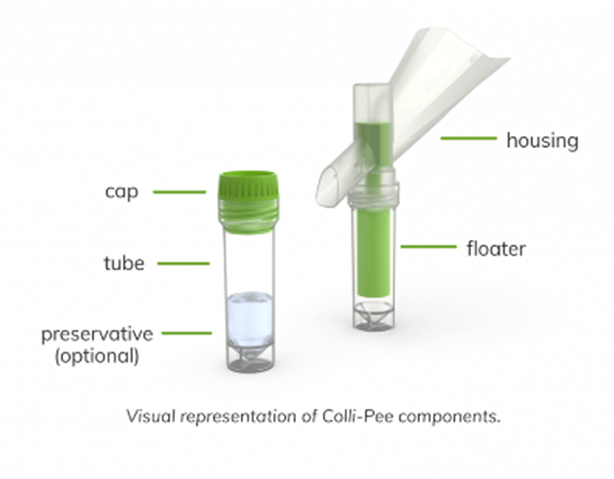
- Unscrew the tube cap (C).
- Screw or push the collector tube (D) on the Colli-Pee housing.
- Collect urine: The device will automatically collect the correct volume.
- There is no need to interrupt the urine stream. Excess urine that no longer fits into the collector tube will flow into the toilet via the other end of the housing.
- Disconnect the collector tube from the Colli-Pee housing.
- Close the tube tightly. Tube cap (C) should be screwed on tightly.
- Dispose the Colli-Pee device according to local regulations for plastic waste.
- Wash your hands.
Product Overview
Colli-Pee is available in various configurations and volumes. As a neat product and prefilled with a preservative.
Find out more about the available options for the CE-IVD/FDA listed versions of the Colli-Pee devices.
Overview neat Colli-Pee products
| Name | Colli-Pee® FV-5004 | Colli-Pee® FV-5010 | Colli-Pee® FV-5020 | Colli-Pee® FV-5040 |
|---|---|---|---|---|
| Reference | N00290 | N00309 | N00055 | N00269 |
| Total volume | 3-5 mL | 9-12 mL | 16.5-23 mL | 33.5-42.5 mL |
| Preservative | NA | NA | NA | NA |
| Storage temperature | 0-40°C | 0-40°C | 0-50°C | 0-50°C |
| Shelf-life | 5 years | 5 years | 5 years | 5 years |
| Housing-tube interface | Screw thread | Push fit | Screw thread | Push fit |
*Some Novosanis products are under development or not available in all geographic regions. Contact us to know the registration status in your region.
Colli-Pee and urine preservation
Urinary analytes are susceptible to enzymatic and chemical degradation. Additionally, in the time between urine collection and processing, analytes can degrade. Consequently, methods to stabilize urine samples are necessary. Novosanis offers several preservatives that allow for sample stability, shipping, and storage at room temperature. A preservative would allow for at-home collection and facilitate large-scale recruitment.
The Colli-Pee device architecture enables immediate mixing of the sample with a urine preservative, improving stability of the urine specimen.
The combination of the Colli-Pee platform with a preservative for urine stabilization can be game-changing. The use of a preservative enables at-home sample collection in the field of urine-based diagnostic testing.
Colli-Pee can be prefilled with Novosanis’ UCM®, to allow preservation of DNA in urine. Colli-Pee containing UCM® has been CE-IVD marked, and it is registered in several countries outside Europe.
To extend the preservation coverage to other analytes (RNA, EV, cfDNA) our newest product Colli-Pee® UAS™ has been developed. Colli Pee UAS™ is available since early 2022 in a Large Volumes format for research use only (RUO) (6).
Optimal preservative to sample ratio depends on the type of preservative, our data shows:
- UCM® – 1:3 Preservative to sample ratio for optimal preservation performance
- UAS™ – 2:5 Preservative to sample ratio for optimal preservation performance
The Colli-Pee device filled with preservative is available in various configurations and volumes. Find out more about the available options.
Overview Colli-Pee containing UCM preservative products
| Name | Colli-Pee® UCM® FV-5003b | Colli-Pee® UCM® FV-5004 | Colli-Pee® UCM® FV-5010 | Colli-Pee® UCM® FV-5020 |
|---|---|---|---|---|
| Reference | N00383 | N00260 | N00327 | N00176 |
| Total volume | 1.7-2.9 mL | 3-5 mL | 9-12 mL | 16.5-23 mL |
| Preservative | UCM 0.9 mL | UCM 1.4 mL | UCM 3.4 mL | UCM 7 mL |
| Pre-usage storage | 9 months at 2-30°C | 2 years at 2-30°C | 2 years at 2-30°C | 2 years at 2-30°C |
| Sample storage | 14 days at 4°C 7 days at room temperature (RT) |
14 days at 4°C 7 days at RT |
14 days at 4°C 7 days at RT |
14 days at 4°C 7 days at RT |
Overview Colli-Pee containing UAS preservative products
| Name | Colli-Pee® UAS™ FV-5040 | Colli-Pee® UAS™ FV-5010-RUO |
|---|---|---|
| Reference | N00342 | N00345_RUO |
| Total volume | 33.5-42.5 mL | 13 mL |
| Preservative | UAS 11.5 mL | UAS 3 mL |
| Pre-use storage | 1 year at 15-30°C | 1 year at 15-30°C |
| Sample storage | 7 days at RT | 7 days at RT |
| Housing-tube storage | Push fit | Push fit |
*Some Novosanis products are under development or not available in all geographic regions. Contact us to know the registration status in your region.
Home collection
Home-based collection kits

Novosanis can provide Colli-Pee in an envelope for easy home-based urine collection. The kit allows postal delivery at home, as well as post-collection postal shipment of the urine sample to the lab.
Why is home-collection needed?
More people want options for managing their health in the comfort of their home. Health care provider-based testing is associated with excessive costs, requires essential healthcare worker resources, and involves risks of infection to the patient and staff.

The need for additional decentralized testing capacity is now more apparent than ever. Currently, there is a lack of user-friendly and high-quality urine sampling devices for use at home that are available in a simple and cost-efficient way.
Colli-Pee postal kits provide a complete solution that includes the device for collection and postal kits provide a complete solution that includes the device for collection and postal packaging for safe storage and transportation.
Additionally, the COVID-19 pandemic has significantly changed the perception of diagnostics in general, especially home-based devices as well as self-testing. Major shifts in healthcare procedures have emerged as facilities adapt at-home testing methods to supplement in-person visits to a health care provider.
*Some Novosanis products are in development or not available in all geographic regions. Contact us to know the registration status in your region.
Colli-Pee Postal Kits for home collection
An innovative urine collection solution for home-based sampling
The Colli-Pee Postal Kit is an accessory to the Colli-Pee device, which aims to leverage postal services, for the distribution of the device to the patient and/or the return of the collected urine sample.
Colli-Pee Postal Kit configurations

The Colli-Pee Postal Kit is available in different configurations under UN3373 compliancy. Postal Kit Accessories with one or more of the following items, depending on the needs of the customer:
- Distribution envelope
- Return envelope
- Safety bag/ biohazard bag
- Absorbing tissue
- Return address label
Quality
The Novosanis quality management system is compliant with the requirements of ISO13485 quality standards for manufacturers of medical devices. ISO 13485;2016 is an FDA recognized standard and deemed equivalent US FDA Quality Systems Regulations (QSR).

- Meers et al., 2019, EUROGIN Poster: Standardized and volumetric collection of first-void urine for detection of STIs and HPV: A comparison between Colli-Pee and a standard urine cup (EUROGIN 2019) https://novosanis.com/sites/default/files/poster/pdf/Meers%20et%20al_Eurogin%202019_Volumetric%20Collection_0.pdf?1669200547
- Brian M Nolen 1 , Anna E Lokshin Int J Biol Markers . 2011 Jul-Sep;26(3):141-52. PMID: 21928247; PMCID: ; DOI: 10.5301/JBM.2011.8613
- A Vorsters 1 , J Van den Bergh, I Micalessi, S Biesmans, J Bogers, A Hens, I De Coster, M Ieven, P Van Damme, Eur J Clin Microbiol Infect Dis. 2014 Nov;33(11):2005-14.; PMID: 24916950; DOI: 10.1007/s10096-014-2147-2
- Irith De Baetselier 1 , Hilde Smet 1 , Said Abdellati 1 , Bénédicte De Deken 1 , Vicky Cuylaerts 1 , Thijs Reyniers 2 , Bea Vuylsteke 2 , Tania Crucitti 1 , BMJ Open. 2019 Apr 3;9(4):e028145. PMID: 30948618 ; PMCID: PMC6500257; DOI: 10.1136/bmjopen-2018-028145
- Téblick, L.; Van Keer, S.; De Smet, A.; Van Damme, P.; Laeremans, M.; Rios Cortes, A.; Beyers, K.; Vankerckhoven, V.; Matheeussen, V.; Mandersloot, R.; Floore, A.; Meijer, C.J.L.M.; Steenbergen, R.D.M.; Vorsters, A. Impact of Collection Volume and DNA Extraction Method on the Detection of Biomarkers and HPV DNA in First-Void Urine. Molecules 2021, 26, 1989. https://doi.org/10.3390/molecules26071989
- Arora et al., (EACR 2022) Poster Stabilization and characterization of urinary cell-free DNA to facilitate home-based testing. https://novosanis.com/sites/default/files/poster/pdf/Poster%20Amit%20-%20Stabilization%20and%20characterization%20of%20urinary%20cell-free%20DNA%20to%20facilitate%20home-based%20testing.pdf?1669200829