Cervical Cancer
You are here
- Introduction
- Symptoms
- Causes and risk factors
- Screening and Detection
- Cervical cancer and urine detection
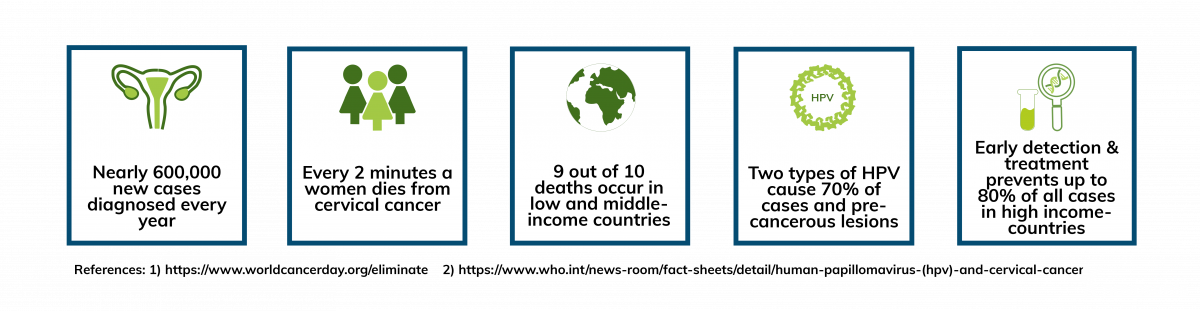
Introduction
The cervix is a tissue that connects the uterus and the vagina. The cervix has two different components which are made up of different kinds of cells:
- Endocervix: The region closest to the uterus is covered with glandular cells
- Exocervix: The region closest to the vagina is covered with squamous cells
The region where both cell types come together is called the transformation zone and is the place where most cancers develop.
Various strains of Human Papillomavirus (HPV) play a major role in cervical cancer development. In particular, high-risk types of HPV are responsible for causing over 99 percent of cervical cancer cases.
Screening as well as the HPV vaccination are methods to reduce the risk of developing the cancer type (1)(2).
Symptoms
Early signs of cervical cancer and/or pre-cancer are difficult to spot. The most common symptoms are (1):
- Abnormal vaginal bleeding after sexual intercourse, between periods or after menopause
- Unusual discharge from the vagina
- Pain during sexual intercourse
Causes and risk factors
High-risk HPV, in particular HPV16 and 18 are the main cause of cervical cancer. HPV infections are contagious and can spread from one person to another through close contact, including sexual activity. Although HPV infections are very common, in most cases the body clears the infection naturally, causing no harm to an individual. However, persistent infections especially of high-risk types can become chronic and can cause various cancers(1) (3) (4).
There are several factors that can increase a person’s risk of developing cervical cancer. Some include (1)(2):
- Lifestyle factors: Smokers are twice as likely to develop cervical cancers than non-smoking women.
- Infection with other Sexually Transmitted Infections (STI): A previous or current chlamydia infection increases the risk of cervical cancer in women.
- Family history: The chance of developing cervical cancer is higher if a family member (i.e. mother or sister) had the disease.
- Multiple full-term pregnancies: Having 3 or more full-term pregnancies or being younger than 17 at your first full-term pregnancy increases the risk of developing cervical cancer.
Screening and detection
If detected at an early stage, cervical cancer is one of the most preventable cancer types. Regular examinations to monitor healthy individuals are recommended every three to five years for women between the ages of 25 and 65.
Current screening methods are primarily cytology-based through detection of abnormal cells (5):
- Pap test: Scraping cells from the cervix to check for abnormalities
- HPV DNA test: Collecting cells from the cervix to check for infection with any of the types of HPV
Despite the benefits of screening, participation remains low in many areas. Some barriers include the methods are perceived invasive, time-consuming and are clinician dependent. Additionally, women in some areas are reluctant to have a pelvic examination (6).
Consequently, finding non-invasive and easy self-collection sampling method can improve participation in cervical screening programs. Urine as a sample type to detect HPV DNA is promising (6)(7).

Read our blog about urine as a preferred sample type for cervical cancer screening
Read our blog about the impact of COVID-19 on cervical cancer screening
Cervical cancer and urine detection
Urine offers several benefits for cervical cancer screening. A urine sample can easily be taken at home, is non-invasive, thereby does not interfere with the natural history of the infection. Additionally urine collection can be performed independently, addressing many of the concerns with the current cytology-based methods8. Initial urine flow contains higher concentrations of HPV DNA and several studies have shown the benefits of first-void urine in HPV detection(9). Colli-Pee offers a user-friendly method to capture a first-void urine, making sample collection easier.
A recent study conducted in France showed the benefits of urinary HPV DNA testing in reaching women who traditionally did not attend cervical cancer screening clinics(10).
Alongside screening, vaccination is vital in the fight against cervical cancer. The HPV vaccine is routinely offered to girls aged 12 to 16 in many countries, and protects women against types of HPV infections. Recent vaccine impact studies conducted by the World Health Organization in Rwanda and Bhutan successfully used urine collected through Colli-Pee to monitor the impact of HPV vaccination (11)(12).
Listen to our podcast on urine sampling for HPV testing
References:
1. https://www.cancer.org/cancer/cervical-cancer.html
2. PubMed PMID: 28689156.
3. PubMed PMID: 28369882
4. PubMed PMID: 25824730.
5. https://www.mayoclinic.org/diseases-conditions/cervical-cancer/diagnosis...
6.PubMed PMID: 23618210
7.PubMed PMID: 27417431.
8.PubMed PMID: 25664398
9. PubMed PMID: 30452931.
10.PubMed PMID: 25964233.
11.PubMed PMID: 26991686.
12.PubMed PMID: 29571973
