Does volume matter? Impact of collection volumes on detection of biomarkers and HPV DNA in first-void urine
You are here

The study by Laura Téblick (University of Antwerp, Belgium) investigated whether varying first-void urine collection volumes, including 4, 10 and 20 mL collected with Colli-Pee®, affects the detection of human and viral endpoints (i.e., glyceraldehyde 3-phosphate dehydrogenase (GAPDH), β-actin (ACTB), β-globin (HBB) and Human Papillomavirus (HPV).
The results showed that all three different first-void urine collection volumes with UCM allow for biomarker detection.
- Human Papillomavirus (HPV) can be detected in first-void urine
- Methods to optimize first-void urine collection
- Study summary: Impact of different urine volumes on detection of biomarkers and HPV
Human Papillomavirus (HPV) can be detected in first-void urine
First-void urine, also known as first-catch or first-pass urine is collected at any time of the day and is typically referred to the first 20 mL of urine flush. This fraction of urine has shown to be a suitable sample type for detection of human papillomavirus (HPV) DNA, a major cause of cervical cancer.
Urine is considered the preferred choice of self-sampling compared to other available methods used for cervical cancer screening. At-home urine self-sampling also offers the opportunity to reach more women for screening, an added benefit in the current COVID-19 pandemic, where healthcare systems are under pressure.
Methods to optimize first-void urine collection
To use first-void urine as a liquid biopsy for cervical cancer screening, methods to optimize sample collection and DNA extraction are necessary. While first-void urine is known to contain higher concentrations of HPV DNA, the ideal urine volume, which will provide optimal concentration of HPV and human DNA for accurate detection of clinically relevant high-risk HPV infections is not yet fully known.
The currently used first-void urine collection device developed by Novosanis, Colli-Pee® containing UCM, collects the first fraction of urine in a total volume of 20 mL. Colli-Pee® Small Volumes* can allow urine collection of 4 mL and 10 mL.
Study summary: Impact of different urine volumes on detection of biomarkers and HPV
Aim
To better understand the effect of first-void urine collection volumes on detection of human and viral endpoints, the study investigated urine collected with Colli-Pee® variants, including 4, 10 and 20 mL. The research also evaluated different DNA extraction protocols and introduced a universal non-human control (i.e., DNA from phocine herpesvirus 1 (PhHV-1)) in the urine preservative to monitor sample collection, transport, storage, and DNA extraction.
Methods
In the study, 25 women diagnosed with high-risk HPV, home-collected three consecutive samples using different Colli-Pee® variants. Urine samples were sent back to the lab via postal mail where they were aliquoted and stored at −80 °C prior to further analysis. Each collector tube was prefilled with UCM spiked with PhHV-1 DNA as internal control.
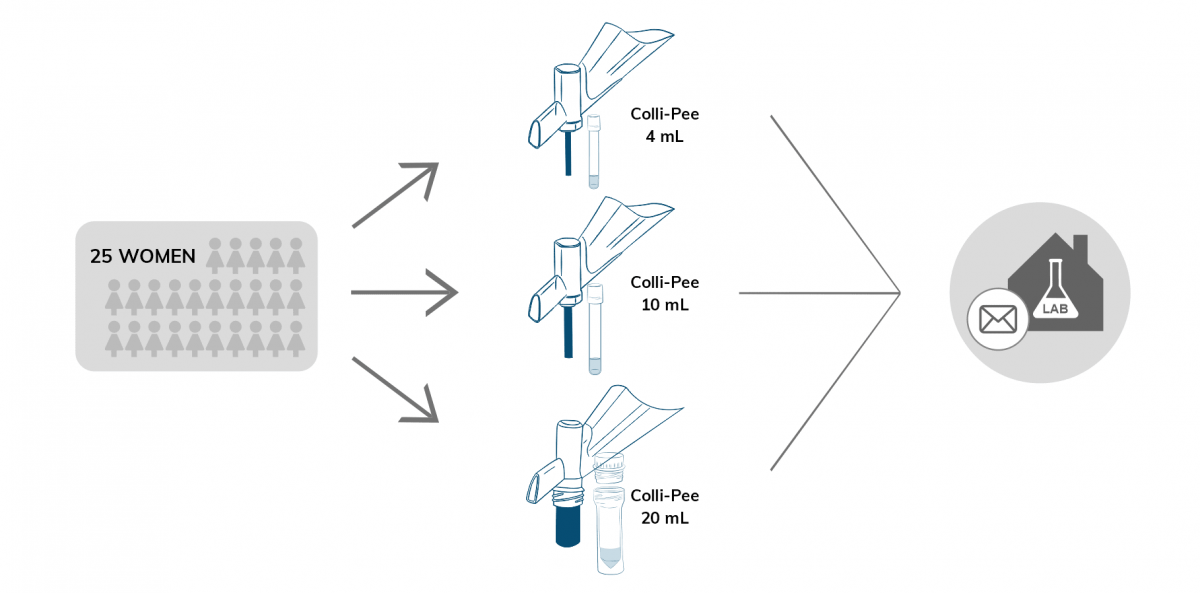
Results
Overall, a good agreement between the three different first-void urine collection volumes was found for human and HPV endpoints. Consequently, the Colli-Pee® Small Volumes 10 mL variant was selected for the remainder of the study as it is compatible with high-throughput instruments and postal delivery, offering potential for high-throughput screening and home-based sample collection. Contrastingly, significant variations in yield for human endpoints were observed for different DNA extraction methods.

Read more about the study in the publication
Learn more about the Colli-Pee® variants
Read more about HPV detection using urine as a sample type
*Some Novosanis products are in development or not available in all geographic regions. Contact us to know the registration status in your region.
