Highlights of 2021: Publications reporting HPV detection in first-void urine collected with Colli-Pee
You are here

Highlights of 2021: Publications reporting HPV detection in first-void urine collected with Colli-Pee®
The number of scientific and peer-reviewed publications highlighting the role of first-void urine in the detection, screening, and research of HPV has increased with time. In this blog post, we recap and summarize the 2021 peer-reviewed publications that used Novosanis’ Colli-Pee® for HPV-related research.
Significant increase in publications linking HPV and urine
Human Papillomavirus (HPV), a common sexually transmitted infection, is linked to various cancers, including cervical cancer. Given this association, methods to detect HPV are important in cancer research.
Urine, in particular, first-void urine (first 20-30 mL of urine flow), is an exciting sample type in the field as it contains higher concentrations of HPV DNA than subsequent fractions. Additionally, urine sampling is easy, non-invasive and suitable for home collection, making the specimen type even more attractive (1,2).
The number of scientific and peer-reviewed publications focusing on the association between urine and HPV has increased overtime. A quick PubMed search has shown a 50% increase in urine/HPV publications from 2014 to 2021.

In particular, publications mentioning Novosanis' Colli-Pee® device for HPV-related research has also increased over the years. In 2021, 7 peer-reviewed publications researched first-void urine collected with Colli-Pee® for HPV detection and monitoring.
Colli-Pee® and HPV detection in first-void urine
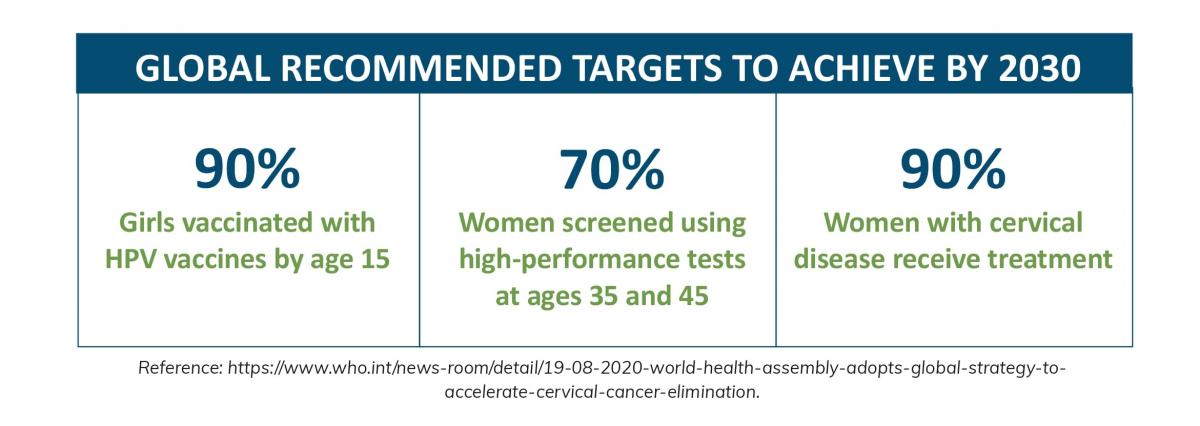
To reduce preventable cervical cancer deaths, methods to improve the uptake of HPV vaccination and cervical cancer screening are necessary. The WHO aims to achieve global targets by 2030 to eliminate the cancer type (3).
Novosanis has been investigating HPV detection in urine for various applications including cervical cancer screening. Our Colli-Pee® device allows for standardized and volumetric at-home collection and stabilization of first-void urine. The device architecture enables immediate mixing of the urine sample with a preservative, improving stability of the sample and its analytes, including HPV.
The 2021 publications using Colli-Pee® for HPV-related research can be grouped together into different themes. These include sample preference, clinical validation, triage and vaccine impact studies.
Below are some of our takeaway messages in each group.
Sample preference:
Given the challenges women face with a Pap smear, there’s a clear need to improve cervical cancer screening participation. Self-sampling devices have been recommended, which offer better acceptance. Women have shown a significantly higher preference to urine as a self-sampling method for HPV testing in comparison to vaginal self-sampling.
- Cervical cancer screening using HPV tests on self-samples: attitudes and preferences of women participating in the VALHUDES study (De Pauw H et al. 2021)
Clinical validation:
First-void urine is a promising alternative to cervical samples for HPV testing and has shown similar clinical accuracy for CIN2+ detection. This offers potential to widen screening strategies and reach un(der)-screened women.
- A Pilot Study of Human Papillomavirus Detection in Urine Using a Novel Nucleic Acid Amplification Test (Marcus JZ et al. 2021)
- A Randomized Comparison of Different Vaginal Self-Sampling Devices and Urine for Human Papillomavirus Testing-Predictors 5.1 (Cadman L et al. 2021)
- Clinical and analytical evaluation of the RealTime High Risk HPV assay in Colli-Pee collected firstvoid urine using the VALHUDES protocol (Van Keer S et al. 2021)
A study also investigated whether varying first-void urine collection volumes, including 4, 10 and 20 mL collected with Colli-Pee®, affects the detection of various biomarkers including HPV. The results showed that all three different first-void urine volumes containing UCM allow equally good biomarker detection.
- Impact of Collection Volume and DNA Extraction Method on the Detection of Biomarkers and HPV DNA in First-Void Urine (Téblick L et al. 2021)
Triage:
As not all HPV infections lead to cervical cancers, identifying only those HPV positive women with clinically relevant infections is important for follow-up. Molecular-based triage methods on HPV positive women using self-collected urine samples can help avoid overreferral and overtreatment.
- Triage of human papillomavirus infected women by methylation analysis in first void urine (Van Keer S et al. 2021)
Vaccine Impact:
The benefits of HPV vaccination for women in cervical cancer prevention is well-researched. HPV vaccination programs are implemented in many countries since 2010, however, uptake is yet limited in many areas (4).
First-void urine can be used to monitor HPV vaccination impact. Data collected from Rwanda and Bhutan show that Colli-Pee® collected first-void urine is a well-accepted sample type and can be used to monitor the effectiveness of the vaccine.
- Impact of Human Papillomavirus Vaccination, Rwanda and Bhutan (Baussano I et al. 2021)
Future perspectives
Interest in first-void urine as sample type for HPV-related research continues to grow. The number as well as range of scientific publications in the field has been following an upward trend. We are excited to be part of this growing space and look forward to what 2022 has in store!
Learn more about our research and publications.
- https://novosanis.com/human-papillomavirus-hpv#3
- https://novosanis.com/our-products/colli-pee-and-first-void-urine
- https://novosanis.com/search/node/global%20targets
- https://novosanis.com/search/node/vaccination%20impact
“The information presented in this blog is made available for general information purposes and may include content provided by third parties. We do not warrant the accuracy, completeness or usefulness of this information and disclaim all liability arising from reliance on it. The statements and opinions expressed in these materials, other than the content provided by us, are solely those of the person or entity making the statements."
