Human Papillomavirus (HPV)
You are here
- What is Human Papillomavirus (HPV)
- HPV and cervical cancer
- First-void urine for HPV-based cervical cancer screening
- HPV and the Colli-Pee device
- HPV prevention through vaccination
What is Human Papillomavirus (HPV)
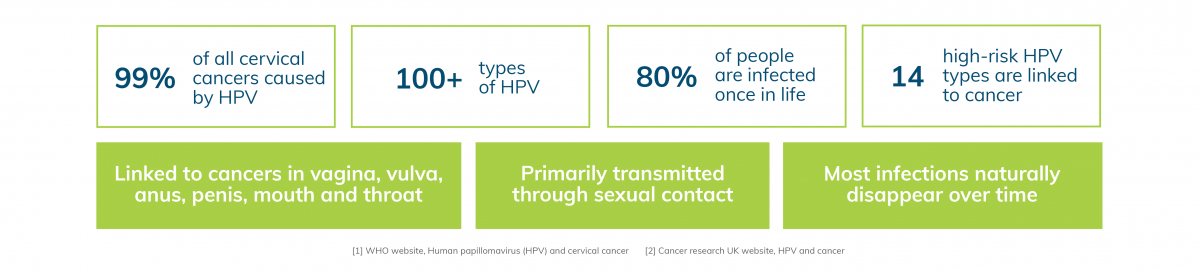
Human Papillomavirus (HPV) is the most common sexually transmitted infection (STI), which affects the skin and/or lining of cells inside the body. In most people, the infection causes no harm, and disappears naturally overtime1.
However, persistent HPV infections can lead to long-term damage. Additionally, strains of HPV, such as high-risk types can cause changes in cell DNA, leading to various cancers. HPV16 and 18 are known to be a major cause of cervical cancer in women. A high percentage of penile and anal cancers have also been linked to HPV16. In certain high-risk groups, such as men who have sex with men (MSM) as well as HIV-positive individuals, are more likely to develop HPV persistent infections1.
HPV and cervical cancer
Cervical cancer is the fourth most common cancer worldwide, leading to over 300,000 deaths per year. While screening can reduce 50% of premature deaths, non-attendance rates are high. On average 40% of women living in developed countries and 80% in developing countries are not participating in regular screening2.
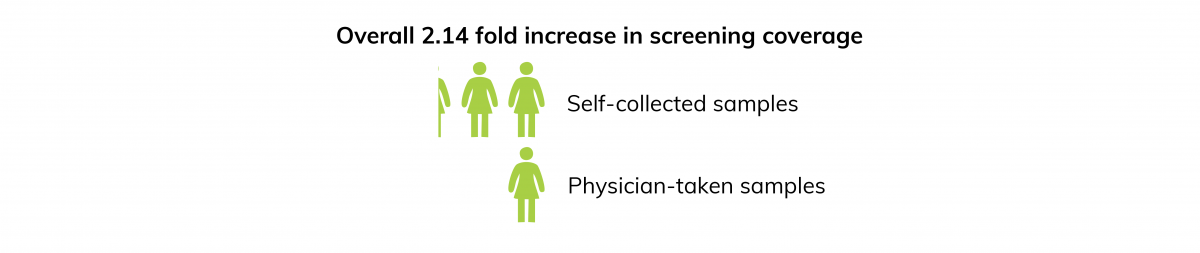
The golden standard to screen for pre-cancerous lesions has been cytologic evaluation through cliniciantaken Pap smears. Reasons for reluctance to gynecological examinations are the relative invasive character of cervical sampling, ethnicity and culture, lack of time and the need to visit a clinician2. Given the challenges women face with Pap smear, ways to improve screening are required. Selfcollection techniques have been recommended, which offer better acceptance. Self-sampling methods for cervical cancer screening have shown to be more effective and efficient to increase participation in screening programs. Studies have shown that women prefer urine as a sample type for hrHPV testing in comparison to brush-based cervical-vaginal self-sampling methods2,3.
First-void urine for HPV-based cervical cancer screening
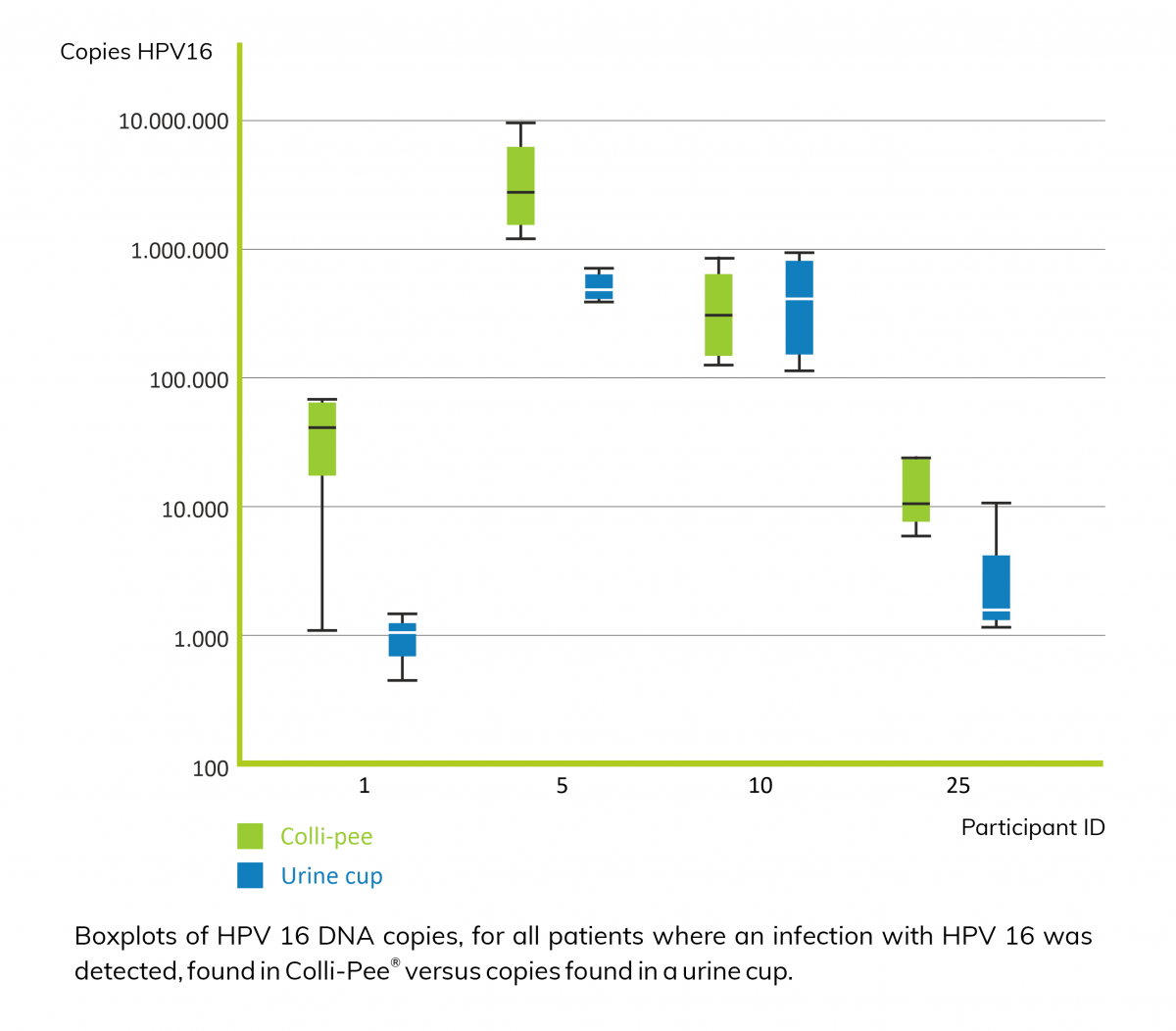 First-void urine (first 20-30 mL of urine flow) contains washed away mucus and debris from exfoliated superficial cell layers of a cervix carcinoma, and higher concentrations of HPV DNA than midstream urine2.
First-void urine (first 20-30 mL of urine flow) contains washed away mucus and debris from exfoliated superficial cell layers of a cervix carcinoma, and higher concentrations of HPV DNA than midstream urine2.
Consequently, it is critical to capture the appropriate urine fraction for improved sensitivity. However, collecting a first-void urine sample with a standard urine cup can be awkward, messy and inconvenient for the user. This is where Novosanis' Colli-Pee®, a user-friendly, self-sampling urine-collection device fits in, as it allows for volumetric and standardized collection of first-void urine. Moreover, Colli-Pee® outperforms a regular urine cup with regards to the number of both human and HPV DNA copies found in urine2.
HPV detection in Colli-Pee collected first-void urine samples
Proof of concept studies with Commercially available diagnostic assays
Several pilot studies confirmed feasibility of HPV DNA detection in first-void urine with commercially available diagnostic assays for automated screening (Roche Cobas HPV, BD Onclarity HPV, Aptima HPV Hologic Panther, Cepheid Xpert HPV) or genotyping (Genefirst Papilloplex HR-HPV, Anyplex II HPV HR Seegene, Fujirebio Innolipa, High+Low Papillomastrip Operon)2.
Clinical performance and ongoing projects
Several clinical trials have been set-up, in more than 2500 women referred to colposcopy, to address the performance of Colli-Pee® collected first-void urine to other self-sampling devices for HPV detection, and understand its potential in cervical cancer screening2.
- EVAH study showed that first-void urine collected with Colli-Pee® offers nearly perfect sensitivity, similar to that of a clinician-taken smear and a vaginal swab self-sample for HPV detection in women with CIN2+ (cervical intraepithelial neoplasia grade 2+)
- VALHUDES aims to evaluate the clinical accuracy of first-void urine collected with Colli-Pee®, the performance compared to other sample types and in different commercially available assays. Protocol
- Predictors 5.1 aims to evaluate the clinical accuracy of first-void urine collected with Colli-Pee®, the performance compared to other sample types and in different commercially available assays.
- CASUS aims to offer a molecular screening solution for cervical cancer screening based on first-void urine collected with Colli-Pee®. It also aims to enable triage on the same urine sample based on methylation markers.
HPV prevention through vaccination

Alongside effective screening, the HPV vaccination is also critical in the fight against HPV-related cancers, including cervical cancer.
National HPV vaccination programs have been implemented in many high-income countries since 2010. Developing countries, such as Bhutan (2010) and Rwanda (2011) are also among the first countries in Asia and Africa respectively to roll-out HPV vaccination programs for schoolgirls. Yet, HPV vaccines are still not widely available. Vaccine impact studies that highlight the benefits and potential of vaccination on a population can create more awareness and reach a wider group. Urine, as a sample offers an easy and simple approach to monitor the effectiveness1.
A study in collaboration with the World Health Organization (WHO) investigated the impact of vaccination against HPV in Rwanda and Bhutan in urine collected with Colli-Pee®. HPV urine surveys were conducted in both countries with around 1,000 girls. The results concluded that HPV presence in urine was associated with sexual activity. In both Bhutan and Rwanda HPV6/11/16/18 prevalence was lower in vaccinated than in unvaccinated participants, highlighting the importance and impact of vaccination in HPV prevention1.
Read more about HPV detection using urine as a sample type
References:
